Answer:
c. 26.9%
Step-by-step explanation:
1st) To calculate the percentage by mass of a solution, it is necessary to use the grams of solue and the grams of solution.
The information given in the exercise is:
- Mass of solute (HCl): 36.0g
- Mass of solvent (water): 98.0g
We need the mass of solution, so it is necessary to add the mass of solute to mass of solvent, that is the mass of solution:
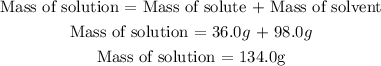
2nd) Now we have to replace the values in the formula to calculate the percentage by mass of the solution:
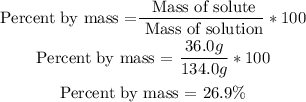
Finally, the percentage by mass of the solution is 26.9%.