ANSWER
The limiting reactant is chlorine
The mass of AlCl3 is 246.41 grams
Explanation
Given information
The mass of aluminum = 94 grams
The mass of chlorine = 197 grams
Firstly, we need to write a balanced equation of the reaction
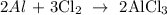
From the reaction above, you will see that 2 moles of aluminum react with 3 moles of aluminum
The next step is to find the number of moles of aluminum and chlorine using the below formula
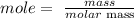
Recall, that the molar mass of aluminum and chlorine is 27 g/mol and 71 g/mol
For aluminum, the number of moles is calculated below as
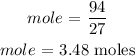
For chloride, the number of moles can be calculated below as
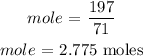
Since we have gotten the number of moles of each reactant, therefore, we can now determine the limiting reactant by dividing the number of moles of each reactant by its coefficient.
The coefficient of aluminum in the reaction is 2 and the coefficient of chloride in the reaction is 3

From the above calculations, you will see that chlorine has the least number of moles which is 0.925 moles.
Therefore, the limiting reactant is chlorine.
The next step is to find the number of moles of aluminum chloride using a stoichiometry ratio.

Hence, the number of moles of aluminum chloride is 1.848 moles
Finally, find the mass of aluminum chloride using the below formula
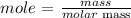
Recall, that the molar mass of aluminum chloride is 133.34 g/mol
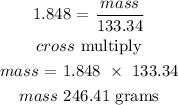
Therefore, the mass of aluminum chloride produced is 246.41 grams