Answer:
4.25 g of magnesium.
Step-by-step explanation:
What is given?
moles of Na2CO3 = 0.0877 moles.
molar mass of Mg (magnesium) = 24.3 g/mol.
Step-by-step solution:
First, let's state the chemical equation between Mg (magnesium) and Na2CO3:
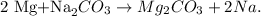
You can see that 1 mol of Na2CO3 reacts with 2 moles of Mg, so let's see how many moles of Mg are being produced by 0.0877 moles of Na2CO3:
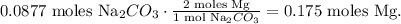
And the final step is to convert from 0.175 moles of Mg to grams using its molar mass. The conversion will look like this:
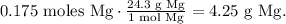
The answer is that there is 4.25 g of magnesium dissolved in the solution of 0.0877 moles of Na2CO.