2 moles of Sn are produced.
From the balanced equation, we can see that 2 moles of hydrogen gas (H2) produce 2 moles of water (H2O) and 1 mole of tin (Sn).
So, with a mathematical Rule of Three we can calculate the number of moles of Sn produced when 4.0 moles of H2 are completely consumed:
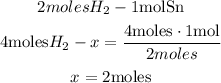
Finally, we found that 2 moles of Sn are produced.