Step 1 - Brief revision of density
Density is defined as the quotient between mass (m) and volume (V):
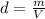
It measures thus the amount of space a gram of some substance would occuppy. A very dense substance will occupy a smaller volume than a less dense one, for the same mass.
Step 2 - Using the formula to solve the problem
The exercise gives us the density of the gasoline, as well as the volume it occupies (3.78 L). Note that the density is given in unities of g/ml. Therefore, the first thing we need to do is to convert L to ml:
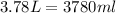
Also note that the tank has 16 gal of volume. Since each gal has 3780 ml, 16 gal is equivalent to:
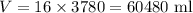
Now let's use the formula we saw in step 1, remembering the density of gasoline is 0.737 g/ml:
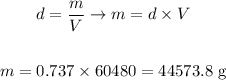
It would be needed thus approximately 44574 g of gasoline to completely fill a 16 gal tank.