Answer:
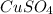
Step-by-step explanation:
Here, we want to get the oxidized species
oxidation is simply the loss of electrons while reduction is the gain of electrons
The Iron atom went from an oxidation state of 0 to +2
Copper went from +2 to 0
What this means is that iron lost electrons, while copper gained electrons
This means copper is the oxidized species