The mass in grams of the sample is 254.956g
- First, we need to know how many moles of Bi are represented in 7.35x10^23 atoms. So, we use the Avogadro's number (6.022x10^23) to make that calculation:
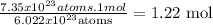
Now, we know that 1.22 moles of Bi have 7.35x10^23 atoms.
- As the molar mass of bismuth (Bi) is 208.98 g/mol we calculate the mass in grams:
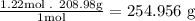
So, the mass in grams of the sample is 254.956g (using three significant figures).