Answer:
a. The equation must be balanced, like this:
![2N\imaginaryI S_2+5O_2\operatorname{\rightarrow}*2N\imaginaryI O+4SO_2.]()
b. The theoretical yield of NiO is 5.08 g.
c. The percent yield would be 95.7%.
Step-by-step explanation:
a. You can identify that this is a double-replacement reaction, so first, by trial and error method, let's write the balanced equation:
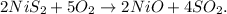
b. Now, we have to find the limiting reactant of 11.2g of NiS2 and 5.43 g of O2. Let's find the number of moles of each of them using the molar mass of each reactant (you can calculate the molar mass using the periodic table). The molar mass of NiS2 is 122.8 g/mol and the molar mass of O2 is 32 g/mol:
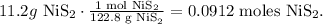
And the moles of oxygen:
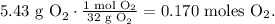
The next step is to find how many moles of NiO are being produce by each reactant using the number of moles. You can realize that in the chemical equation, 2 moles of NiS2 reacted produces 2 moles of NiO, this means that the molar ratio between them is 2:2, simplifying it is 1:1, so 0.0912 moles of NiS2 reacted produces 0.0912 moles of NiO.
Now, for oxygen, you can see in the equation that 5 moles of O2 reacted produces 2 moles of NiO, so the conversion will be:
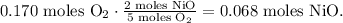
You can see that the limiting reactant in this case, would be oxygen because it produces the smaller number of moles that can produce between the reactants. O2 puts the limit, so the excess reactant would be NiS2. We're producing 0.068 moles of NiO.
To find the theoretical yield of NiO, we have to find the mass of NiO using the molar mass. Remember that the molar mass of NiO is 74.7 g/mol, so the conversion is:
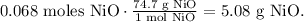
The theoretical yield of NiO is 5.08 g.
c. Finally, to calculate the percent yield, we use its formula:
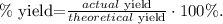
Our actual yield is 4.86 g of NiO and our theoretical yield is 5.08 g of NiO:
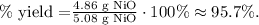
The percent yield would be 95.7%.