The question requires us to calculate the energy, in joules (J), necessary to heat 83.5g of water from 26.7 °C to 75°C.
The heat energy (Q) can be calculated from the mass of the sample (m), its specific heat capacity of the substance (C) and temperature variation (ΔT), as given by the following equation:
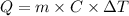
Since the question did not provide a value for the specific heat capacity of water, we'll adopt the value 4.182 J/g.°C. Applying the values provided by the question, we can calculate the heat energy as:
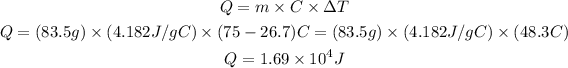
Therefore, the heat energy necessary to heat the amount of water given grom 26.7 to 75°C is 1.69 x 10^4 J (or 16900 J).