ANSWER
The volume of butane in mL is 5967 mL
Step-by-step explanation
Given that;
The mass of butane is 15.45 grams
Follow the steps below to find the volume of butane in mL
Step 1: Write the molecular formula of butane
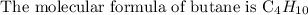
Step 2: Find the molar mass of butane
In the above molecular formula, butane has 4 carbon atoms and 10 hydrogen atoms
Recall, that the mass unit of carbon is 12u and the mass unit of hydrogen is 1.0u
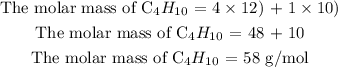
Step 3: Find the mole of butane using the below formula
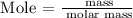
Recall, that the mass of butane is 15.45 grams as provided in the question
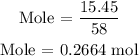
Step 4: Find the volume of butane in mL
Recall, that 1 mole of a gas is equivalent to 22.4L molar volume
Let x represents the volume of butane
From the above data in step 4, we can now find the volume of butane

Hence, the volume of butane in mL is 5967 mL