The question requires us to calculate the rate of change of NO, given that the rate of change of Cl2 is -0.0500 M/s.
The balanced chemical reaction provided is:
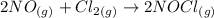
From the stochiometry of the reaction we can say that Δ[NO] = 2/1 Δ[Cl2], thus we can calculate the rate of change of NO as:
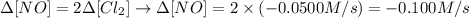
Therefore, the rate of change of NO is -0.100 M/s.