So, 231.16mL are needed.
1st) It is necessary to calculate the moles of H2SO4 that are needed in the 0.150 M solution. We can find the number of moles with a mathematical Rule of Three:
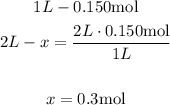
Now we know that are needed 0.3 moles of H2SO4.
2nd) With the molar mass of H2SO4 we can calculate the grams of H2SO4 that are needed. The molar mass of H2SO4 is 98.1g/mol.
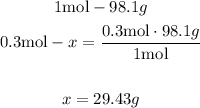
Now we know that are needed 29.43g of H2SO4. This is the amount of solute that it is necessary to make the new solution.
3rd) From the formula of the concentration %w/w, we can calculate the mass of solution that it is needed:
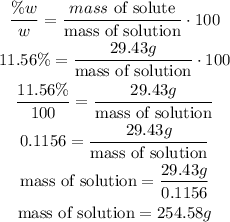
So, it is necessary 254.58g of the 11.56% solution of H2SO4.
4th) Finally, it is necessary to use the density value to convert grams into milliliters:

So, 231.16mL of the 11.56%w/w solution of H2SO4 are needed.