Step 1
The equation to calculate ΔH (change of enthalpy) is:
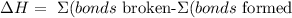
(In the absence of standard formation enthalpies)
--------------------
Step 2
The reaction provided:
CH4 + 4 Cl2 => CCl4 + 4 HCl
--------------------
Step 3
Procedure: from step 1,
ΔH = [H(4H-C) + H(Cl-Cl)] - [H(4C-Cl) + H(4H-Cl)]
ΔH = [(4x411 + 4x242) - (4x327 + 4x427)] kJ/mol = - 404
ΔH = - 404 kJ/mol < 0 => Exothermic
Answer: The reaction is exothermic because the total bond energy of the reactants is less than the total bond energy of the products.