1) Balance the chemical equation.
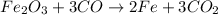
2) Convert grams of Fe into moles of F2.
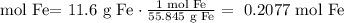
0.2077 mol Fe was produced.
3) Moles of CO needed to produce 0.2077 mol Fe.
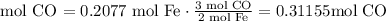
4) Convert moles of CO into molecules of CO
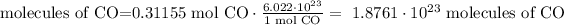
1.88*10^23 molecules of carbon monoxide are needed to react with excess iron (III) oxide.
.