Answer
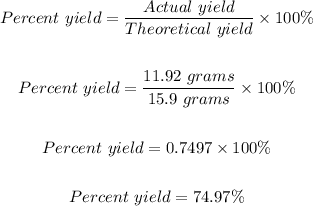
The percent yield = 74.97%
Step-by-step explanation
A reaction produced 11.92 grams of O₂ implies the actual yield = 11.92 grams of O₂
When it was calculated that it would produce 15.9 grams of O₂ implies the theoretical yield = 15.9 grams of O₂
Therefore, the percent yield is