We must take into account the definition of pH, and this represents the concentration of H+ ions([H+]). It can be defined with the following equation:
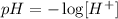
To determine how many times the acid is stronger than the tomato, we must calculate the concentration of H+ ions for each pH value, to do so we clear [H+] from the previous equation:
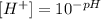
Now we substitute each pH value into the equation:
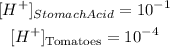
Therefore we will have to:
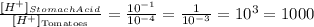
Therefore, stomach acid will be 1000 times stronger than the tomatoes.