Given:
The charge on the object is
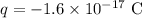
Required: Number of electrons
Step-by-step explanation:
The number of electrons can be calculated using the quantization of charge
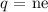
Here, n is the number of electrons
e is the charge on the electron whose value is
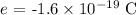
On substituting the values, the number of electrons will be
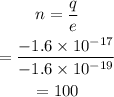
Final Answer: The object has an excess of 100 electrons.