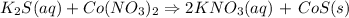1. Synthesis reaction : there is only 1 product formed from 2 or more reactant
E.g:
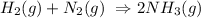
2. Decomposition : reaction that occurs in presence of UV light and only 1 reactant that decomposes into 2 or more products.
E.g:
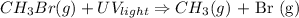
3. Single displacement : reaction that occurs when 1 reactant displaces other reactant from its compound:
E.g:
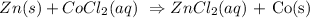
4. Double displacement : reaction that occurs when both reactant displaces each other.
E.g :