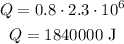In order to calculate the amount of energy needed, we can use the formula below:
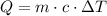
Where Q is the amount of energy in Joules, m is the mass in kg, c is the specific heat coefficient and DeltaT is the change in temperature.
So, for m = 0.8, c = 4.2*10^3 and DeltaT = 85 (from 15°C to 100°C), we have:
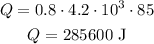
Then, for the energy needed to evaporate the water, we have a similar formula:
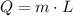
Where L is the latent heat. So, for L = 2.3 * 10^6, we have: