Answer:
The concentration of the
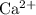 ions in the solution would continue to be
ions in the solution would continue to be
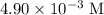 provided that the temperature the solution stays the same.
provided that the temperature the solution stays the same.
Step-by-step explanation:
Normally, evaporating the solvent (e.g., water) from a dilute salt solution would increase the concentration. However, in most cases, there is a limit on the maximum concentration of that solution. One would expect the solute (the salt) to precipitate out of the solution when the solution is saturated with this solute.
However, if the salt solution is saturated to begin with, the solute would start to deposit out of the solution immediately after evaporation starts. If the temperature of the solution stays the same, concentration won't increase at all.
The question states that the calcium sulfate solution in this question is saturated to begin with. Thus, the initial concentration of the saturated solution (
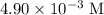 ) would be the maximum concentration that this solution could achieve at this temperature. If temperature stays unchanged, evaporating water out of the solution would deposit calcium sulfate out of the solution; the concentration of the solute would continue to be
) would be the maximum concentration that this solution could achieve at this temperature. If temperature stays unchanged, evaporating water out of the solution would deposit calcium sulfate out of the solution; the concentration of the solute would continue to be
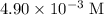 .
.
The concentration of
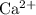 in this solution is directly proportional to the concentration of
in this solution is directly proportional to the concentration of
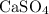 in the solution.
in the solution.
Thus, the concentration of
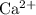 in this solution would not change and continue to be
in this solution would not change and continue to be
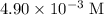 .
.