Answer:
About 4.79 moles of oxygen.
Step-by-step explanation:
We want to determine the amount of moles of oxygen are present in 0.798 moles of Mg(IO₃)₂.
From the chemical formula, we can see that there are six atoms of oxygen for every molecule of Mg(IO₃)₂. Therefore, we can say that there are six moles of oxygen for every mole of Mg(IO₃)₂. This yields the following ratio:
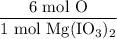
Multiplying this by the initial value yields:
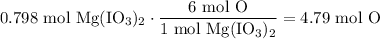
In conclusion, there are about 4.79 moles of oxygen present in 0.798 moles of Mg(IO₃)₂.