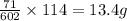Answer:
13.4g
Step-by-step explanation:
we know that:
1 mole = 6.02 × 10²³ atoms
make the unknown number of moles = x
x = 7.1 × 10²² atoms
putting them both together:
1 mole = 6.02 × 10²³ atoms
x = 7.1 × 10²² atoms
Cross multiply:
6.02 × 10²³ x = 7.1 × 10²²
divide both sides by 6.02 × 10²³
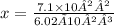
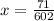
we now have the number of moles of Al₂CO₃
to calculate the grams (mass):
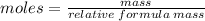
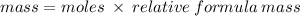
add up all of the atomic masses of Al₂CO₃ to calculate relative formula mass:
(27 × 2) + 12 + (16 × 3) = 114
the grams (mass) of Al₂CO₃: