Answer:
760 mm of Hg
Step-by-step explanation:
If the gases A , B and C are non reacting , then according to Dalton's Law of Partial Pressure the total pressure exerted is equal to sum of individual partial pressure of the gases .
If there are n , number of gases then ,
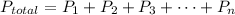
Here ,
- Partial pressure of Gas A = 400mm of Hg
- Partial pressure of Gas B = 220 mm of Hg
- Partial pressure of Gas C = 140mm of Hg
Hence the total pressure exerted is ,
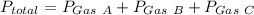
Substitute ,
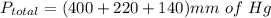
Add ,
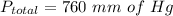
Hence the total pressure exerted by the gases is 760 mm of Hg.
I hope this helps.