Answer:
- Carbon dioxide gas
- Metal salt
- Water
Step-by-step explanation:
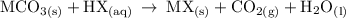
- MCO_3; Can be any metal carbonate such as Magnesium carbonate, Aluminum carbonate.....
- HX; can be any acid such as sulphuric acid, hydrochloric acid, nitric acid.....
- MX; is a metal acidic salt such as magnesium sulphate from magnesium carbonate and sulphuric acid, Aluminum chloride from Aluminum carbonate and hydrochloric acid.