Answer:

Step-by-step explanation:
We are asked to find how many moles of iron are in a sample containing 7.91×10²³ atoms of iron (Fe).
We convert atoms to moles using Avogadro's Number of 6.022 × 10²³. This is the number of particles (atoms, molecules, formula units, etc.) in 1 mole of a substance. In this case, the particles are atoms of iron. There are 6.022 ×10²³ atoms of iron in 1 mole of iron.
Convert using dimensional analysis and set up a conversion factor using the molar mass.
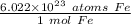
We are converting 7.91 ×10²³ atoms of iron to moles, so we multiply by this value.
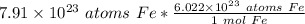
Flip the ratio so the units of atoms of iron cancel.
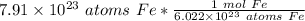
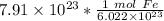
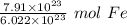
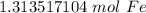
The original value of atoms of iron has 3 significant figures, so our answer must have the same. For the number we found, that is the hundredth place. The 3 in the thousandth place tells us to leave the 1 in the hundredth place.
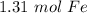
There are approximately 1.31 moles of iron in 7.91×10²³ atoms of iron.