Answer:
From gas laws (pressure law and Boyles law), the pressure exerted by a gas depends on Temperature of the gas and volume of the container.
Step-by-step explanation:
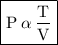
• P → Pressure exerted by the gas.
• T → Temperature of the gas.
• V → Volume of the container.
• from the expression, pressure exerted by the gas is directly proportional to temperature of the gas and inversely proportional to the volume of the container.