Answer:
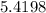 ×
×
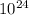 atoms of hydrogen
atoms of hydrogen
Step-by-step explanation:
Firstly, we need to know how many atoms of
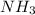 are there in 3 moles of
are there in 3 moles of
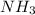 .
.
We know that there is
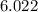 ×
×
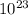 atoms in a mole of any substance.
atoms in a mole of any substance.
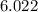 ×
×
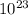 is Avogadro's Constant
is Avogadro's Constant
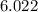 ×
×
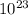 × 3 =
× 3 =
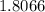 ×
×
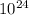
Therefore in 3 moles of ammonia, there are
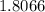 ×
×
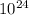 atoms.
atoms.
Next, you should determine how many molecules of an element is present in ammonia.
There is 1 Nitrogen molecule and 3 Hydrogen molecules for every mole of ammonia.
Finally, since we know 3 moles of ammonia contains
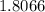 ×
×
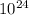 atoms, we just have to multiply accordingly to get the individual amount of atoms of an element a compound has.
atoms, we just have to multiply accordingly to get the individual amount of atoms of an element a compound has.
For Nitrogen :
1 ×
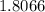 ×
×
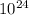 =
=
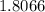 ×
×
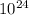 atoms of nitrogen
atoms of nitrogen
For Hydrogen :
3 ×
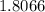 ×
×
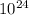 =
=
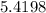 ×
×
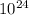 atoms of hydrogen
atoms of hydrogen
Therefore, there is
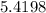 ×
×
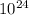 hydrogen atoms in 3 moles of ammonia.
hydrogen atoms in 3 moles of ammonia.
Hope this answer helped!