Answer:
Answer choice B 4,136.42 cubic yards
Explanation:
Volume of a sphere =
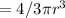
We have r = 11 yards and the sphere was full at the beginning of the day
So volume of water originally stored in this sphere =
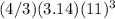 = 5572.45 cubic yards given that
= 5572.45 cubic yards given that
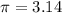
The volume of water lost during the day = 1436.06 cubic yards
So remaining water is
5572.45 - 1436.06 = 4,136.42 cubic yards ie choice B
Note that answer choices A and D are trick answers
Choice A is the original volume of water and choice D is the amount of water lost