Answer: 6 moles of NaCl are produced when 2 moles of sodium phosphate reacts with 3 moles of calcium chloride
Step-by-step explanation:
The balanced chemical equation is:
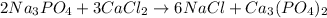
According to stoichiometry :
2 moles of
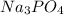 require 3 moles of
require 3 moles of
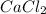
Thus both are limiting reagent as both will limit the formation of product.
As 2 moles of
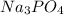 reacts with 3 moles of
reacts with 3 moles of
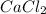 give = 6 moles of
give = 6 moles of
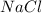
Thus 6 moles of NaCl are produced when 2 moles of sodium phosphate reacts with 3 moles of calcium chloride