Answer:
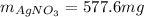
Step-by-step explanation:
Hello there!
In this case, according to the given chemical reaction, it is first necessary to compute the moles of reacting LiOH given its molar mass:
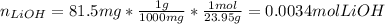
Thus, since there is a 1:1 mole ratio between lithium hydroxide and silver nitrate (169.87 g/mol) the resulting milligrams turn out to be:
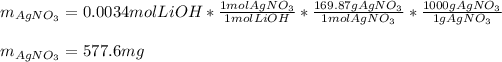
Best regards!