Answer:

Step-by-step explanation:
To convert from molecules to moles, we must use Avogadro's Number: 6.022*10²³. This tells us the amount of particles (atoms, molecules, etc.) in 1 mole of a substance. In this case, the particles are molecules of water.
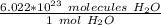
Multiply by the given number of molecules.
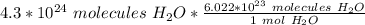
Flip the fraction so the molecules of water cancel.
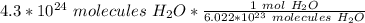
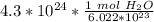
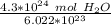
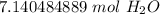
The original measurement of atoms has 2 significant figures ( 4 and 3), so our answer must have the same. For the moles we calculated, that is the tenth place. The 4 in the hundredth place tells us to leave the 1.
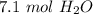
There are about 7.1 moles of water.