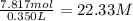Answer: 22.33 M
Step-by-step explanation:
molarity = moles of solute/liters of solution or
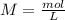
first we have to find our moles of solute (mol), which you can find by dividing the mass of solute by molar mass of solute
mass of solute: 285 g
molar mass of solute: 36.46 g/mol
let's plug it in:
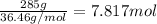
next, we plug it into our original equation: