Answer:
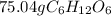
Step-by-step explanation:
Hello!
In this case, since one mol of any atom is related via the Avogadro's number and each mol of glucose has 6 moles of atoms of oxygen, it is possible to obtain the moles of glucose as shown below:

Thus, since the molar mass of glucose is 180.15 g/mol, the mass turns out to be:
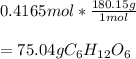
Best regards!