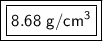
Answer:
Step-by-step explanation:
The density of a substance is its mass per unit volume. The formula for calculating density is:
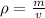
The mass of the sample of metal is 10.5 grams. The volume of the sample of metal is 1.21 cubic centimeters.
Substitute these values into the formula.
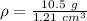
Divide.
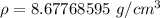
The original measurements of mass and volume have 3 significant figures, so our answer must have the same. For the number we calculated, that is the hundredth place. The 7 in the thousandth place (8.67768595) tells us to round the 7 up to an 8.
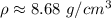
The density of the metal is approximately 8.68 grams per cubic centimeter.