Answer:
Step-by-step explanation:
We are asked to find the specific heat capacity of a liquid. We are given the heat added, the mass, and the change in temperature, so we will use the following formula.
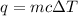
The heat added (q) is 47.1 Joules. The mass (m) of the liquid is 14.0 grams. The specific heat (c) is unknown. The change in temperature (ΔT) is 1.80 °C.
- q= 47.1 J
- m= 14.0 g
- ΔT= 1.80 °C
Substitute these values into the formula.
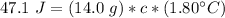
Multiply the 2 numbers in parentheses on the right side of the equation.
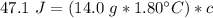
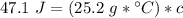
We are solving for the heat capacity of the liquid, so we must isolate the variable c. It is being multiplied by 25.2 grams * degrees Celsius. The inverse operation of multiplication is division, so we divide both sides of the equation by (25.2 g * °C).
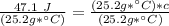
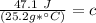
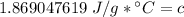
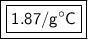
The original measurements of heat, mass, and temperature all have 3 significant figures, so our answer must have the same. For the number we found that is the hundredth place. The 9 in the thousandth place to the right tells us to round the 6 up to a 7.
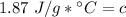
The heat capacity of the liquid is approximately 1.87 J/g°C.