To solve this question, it is important look at the pictue of the atom. The atom has the following properties:
- 17 protons
- 17 electrons
- 18 neutrons
Now, let's judge each affirmation
- This is Chlorine --> Correct
To indentify what element is this atom, we should know what is its atomic number. The atomic number is equal to the number of protons in the atom. In our atom, we have 17 prontos, so, the atomic number is 17.
In the Periodic Table, the atomic number is above of letter that represents the elements. For example, the atomic number of the Helium (H) is 1.
Now, let's look to the Periodic Table and find the element of the atomic number is 17. The element that the atomic number is 17 is Chlorine. So, this affirmation is true.
- This is Bromine --> Incorrect
We saw before that is a atom of Chlorine, because its atomic number is 17. The atomic number of Bromine (Br) is 35.
- The mass of this atom is 35 amu --> Correct
The mass of any atom is equal the sum of the number of protons and the number of neutrons. This happens because the mass of the electrons is negligible. So, let's calculate the mass of our atom:
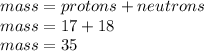
Therefore, the mass of this atom is 35 amu, so the affirmation is correct.
- This atom has an overall charge of 0 --> Correct
Any atom has a overall charge different of 0 if the number of protons and electrons are different.
In our case, the number of protons and alectrons are equals, so the overall charge is 0. So, the affirmation is correct.
- The mass of this atom is 69 amu --> Incorrect
We saw before (third affirmation) that the mass of this atom is 35 amu. So, this is a incorrect affirmation.
I hope I've helped. ^^
Enjoy your studies! \o/