Answer:
The addition of sulfate ions shifts equilibrium to the left.
Step-by-step explanation:
Hello!
In this case, according to the following ionization of strontium sulfate:
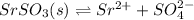
It is evidenced that when sodium sulfate is added, sulfate,
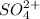 is actually added in to the solution, which causes the equilibrium to shift leftwards according to the Le Ch athelier's principle. Thus, the answer in this case would be:
is actually added in to the solution, which causes the equilibrium to shift leftwards according to the Le Ch athelier's principle. Thus, the answer in this case would be:
The addition of sulfate ions shifts equilibrium to the left.
Best regards!