Answer:

Step-by-step explanation:
Density is the mass per unit volume of a substance. The following formula is used for calculating density.
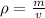
We know the density of aluminum is 2.70 grams per milliliter. The mass is unknown, but we know the piece of aluminum has a volume of 344 milliliters.
Substitute these values into the formula.
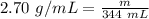
We are solving for the mass, so we must isolate the variable m. It is being divided by 344 milliliters. The inverse operation of division is multiplication. Multiply both sides of the equation by 344 mL.
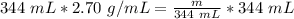
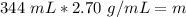
The units of milliliters cancel.
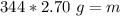
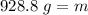
The mass of the piece of aluminum is 928.8 grams.