Answer:
0.322 moles
Step-by-step explanation:
Given Mass, m = 100 g
The molar mass of Ca₃(PO₄)₂
Ca₃(PO₄)₂ = (3 x 40.08) + (2 x 30.97) + (8 x 16.00)
M = 310.18 g/mol
Let there are n number of moles,
n = given mass/ molar mass
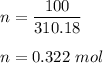
So, there are 0.322 moles.