Answer:

Step-by-step explanation:
We are asked to calculate the liters given the molarity and moles in a solution.
Molarity is a measure of concentration in moles per liter. It is calculated with the following solution:
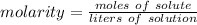
The molarity of the solution is 4.40 molar of potassium chloride (KCl). 1 molar is equal to 1 mole per liter, so the molarity is also 4.40 moles of potassium chloride per liter. There are 0.200 moles of potassium chloride or solute. The liters of solution is unknown, so we can use the variable x.
- molarity= 4.40 moles KCl / L
- moles of solute = 0.200 mol KCl
- liters of solution = x
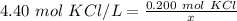
Since we are solving for x, we must isolate the variable. First, cross multiply. Multiply the first numerator by the second denominator, then the first denominator and the second numerator.
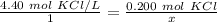
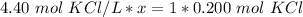
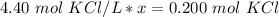
x is being multiplied by 4.40 moles of potassium chloride per liter. The inverse operation of multiplication is division. Divide both sides by 4.40 mol KCl/L
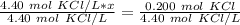
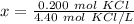
The units of moles of potassium chloride cancel.
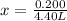
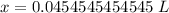
The original measurements of liters and moles have 3 significant figures, so our answer must have the same. For the number we calculated, that is the ten-thousandth place (0.0454545454545). The 5 to the right of this place (0.0454545454545) tells us to round the 4 up to a 5.
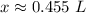
There are approximately 0.455 liters in a 4.40 molar solution with 0.200 moles of solute.