When dissolved in water, many bases release
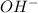 ions
ions
What is a base?
Any material that reacts with hydrogen ions to neutralize an acid is called a basic. The majority of bases are minerals that combine with acids to create salts and water. Metal hydroxides, carbonates, and oxides are examples of bases.An alkali is a base that dissolves in water.
A base releases hydroxide ions when it dissolves in water. The base now has a negative charge due to this. A solution is considered basic if it has a significant concentration of hydroxide ions. In solutions, bases release OH- ions and acids release H+ ions.