Answer:

Step-by-step explanation:
We are asked to find the unknown concentration of the acid given the details of a titration experiment.
A formula for titration is:
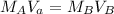
where M is the molarity of the acid of base and V is the volume of the acid or base. There are 56.0 milliliters of the acid (H₃PO₄), but the concentration or molarity of the acid is unknown. There are 88.2 milliliters of the base (KOH) with a molarity of 0.436.
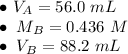
Substitute the values into the formula.
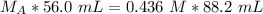
We are solving for the molarity of the acid, so we must isolate the variable
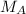
It is being multiplied by 56.0 milliliters and the inverse operation of multiplication is division. Divide both sides of the equation by 56.0 mL.
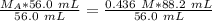
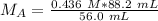
The units of milliliters/mL cancel.
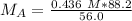
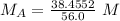
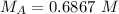
All the original measurements have 3 significant figures, so our answer must have the same. For the number we calculated, that is the thousandths place. The 7 in the ten-thousandths place tells us to round the 6 up to a 7.
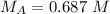
The molarity of the acid is 0.687 M.