Answer:

Step-by-step explanation:
Molarity is a measure of concentration in moles per liter. The formula for calculating molarity is:
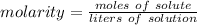
There are 2 moles of a compound or the solute. There are 4 liters of water or the solution.
- moles of solute= 2 mol
- liters of solution = 4 L
Substitute the values into the formula.
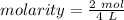
Divide.
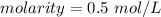
1 mole per liter is equal to 1 Molar, so the solution's molarity of 0.5 mol/L is equal to 0.5 M.
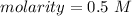
The molarity of the solution is 0.5 Molar and choice B is correct.