Answer:
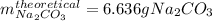
Step-by-step explanation:
Hello!
In this case, since the decomposition of sodium hydrogen carbonate is:
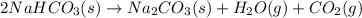
Thus, since there is a 2:1 mole ratio between the sodium hydrogen carbonate and sodium carbonate, and the molar masses are 84.01 and 105.99 g/mol respectively, we obtain the following theoretical yield:
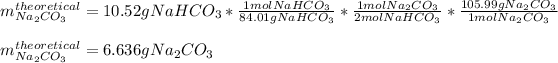
Best regards!