Answer:
The option closest to the percentage yield is option;
d. 81.1
Step-by-step explanation:
The given chemical equation of the reaction is presented as follows;
CaO (s) + H₂O (l) → Ca(OH)₂
The mass of CaO in the experiment, m = 2.00 g
The volume of water with which the CaO was reacted = Excess volume of water
Number of moles = Mass/(Molar mass)
The mass of Ca(OH)₂ recovered, actual yield = 2.14 g
The molar mass of CaO = 56.0774 g/mol
The number of moles of CaO in the reaction, n₁ = 2.00 g/(56.0774 g/mol ≈ 0.036 moles
The molar mass of Ca(OH)₂ = 74.093 g/mol
The number of moles of Ca(OH)₂ in the reaction, n₂ = 2.14 g/(74.093 g/mol) ≈ 0.029 moles
From the given chemical reaction, one mole of CaO reacts with one mole of H₂O to produce one mole of Ca(OH)₂
Therefore, 0.036 moles of CaO will produce 0.036 moles of Ca(OH)₂
Mass = Number of moles × Molar mass
The mass of 0.036 moles of Ca(OH)₂ ≈ 0.036 moles × 74.093 g/mol = 2.667348 grams
∴ The theoretical yield of Ca(OH)₂ = 2.667348 grams
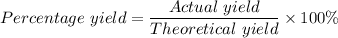
The percentage yield = (2.14 g)/(2.667348 grams) × 100 = 80.23%
Therefore, the option which is closest is option d. 81.1.