Complete Question
Complete Question is attached below
Answer:
Option A
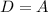 And
And
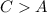
Step-by-step explanation:
From the question, we are told that:
The Chemical Reaction
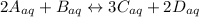
Generally, the equation for Equilibrium constant is mathematically given by
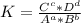
Therefore
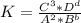
Hence we conculde
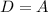 And
And
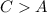
Therefore Option A