Answer:
The volume of the base is 0.05819·x L, where x is the number of moles of base that combines with one mole of sulfuric acid
Step-by-step explanation:
The volume of the sulfuric acid, V = 25.3 mL = 25.3 × 10⁻³ L
The concentration of the sulfuric acid, c = 2.30 M
The concentration of the base,
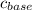 = 1.00 M
= 1.00 M
Let the mole ratio of the acid to base be 1 : x
The number of moles of sulfuric acid present, n = c × V
∴ n = 2.30 M/L × 25.3 × 10⁻³ L = 0.05819 moles
The number of moles of sulfuric acid present, n = 0.05819 moles
1 mole of sulfuric acid combines with x moles of base
Therefore, 0.05819 moles of sulfuric acid will combine with 0.05819·x moles of base
The number of moles of base,
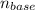 = 0.05819·x moles
= 0.05819·x moles
Therefore, the volume of base,
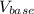 =
=
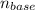 /
/
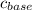
∴
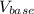 = 0.05819·x/1 ≈ 0.05819·x L
= 0.05819·x/1 ≈ 0.05819·x L
The volume of base,
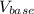 ≈ 0.05819·x L.
≈ 0.05819·x L.