Answer:
The activity of P-32 is 3.7x10¹³ becquerels = 1.0x10³ curies.
Step-by-step explanation:
The activity of P-32 can be calculated with the following equation:
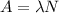 (1)
(1)
Where:
N: is the number of atoms of P-32
λ: is the decay constant
We can find the number of atoms of P-32 as follows:
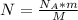 (2)
(2)
Where:
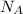 : is the Avogadro's number = 6.022x10²³ atoms/mol
: is the Avogadro's number = 6.022x10²³ atoms/mol
m: is the mass of P-32 = 3.5x10⁻³ g
M: is the molar mass of the radionuclide (P-32) = 32 g/mol
Now, the decay constant is given by:
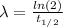 (3)
(3)
Where:
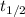 : is the half-life of P-32 = 14.3 days
: is the half-life of P-32 = 14.3 days
Finally, we can find the activity of P-32 by entering equations (2) and (3) into (1):
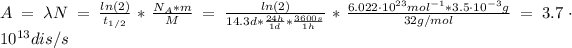
Since a becquerel (Bq) is defined as a disintegration (dis) per second, the activity in Bq is:
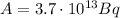
And, since a Curie (Ci) is 3.7x10¹⁰ Bq, the activity in Ci is:
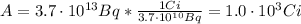
Therefore, the activity of P-32 is 3.7x10¹³ becquerels = 1.0x10³ curies.
I hope it helps you!