Answer:
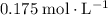 .
.
Step-by-step explanation:
Magnesium chloride and silver nitrate reacts at a
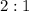 ratio:
ratio:
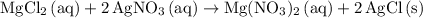 .
.
In reality, the nitrate ion from silver nitrate did not take part in this reaction at all. Consider the ionic equation for this very reaction:
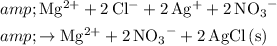 .
.
The precipitate silver chloride
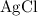 is insoluble in water and barely ionizes. Hence,
is insoluble in water and barely ionizes. Hence,
 isn't rewritten as ions.
isn't rewritten as ions.
Net ionic equation:
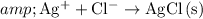 .
.
Calculate the initial quantity of nitrate ions in the mixture.
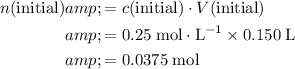 .
.
Since nitrate ions
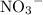 do not take part in any reaction in this mixture, the quantity of this ion would stay the same.
do not take part in any reaction in this mixture, the quantity of this ion would stay the same.
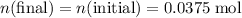 .
.
However, the volume of the new solution is twice that of the original nitrate solution. Hence, the concentration of nitrate ions in the new solution would be
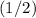 of the concentration in the original solution.
of the concentration in the original solution.
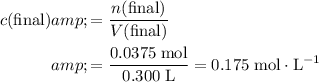 .
.