Answer:
Q = 194.34 J
Step-by-step explanation:
Given that,
100.0 mL of ethyl alcohol is heated from 10.0 C to 35.0 C.
We need to find the amount of thermal energy absorbed by the ethyl alcohol.
The density of ethyl alcohol is 0.790 g/mL.
The formula for heat absorbed is given by :
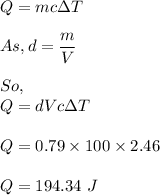
So, 194.34 J of thermal energy is absorbed by the ethyl alcohol.